If you own a healthcare business, you surely understand the complexity and importance of the 340B Drug Pricing Program. As we know, the 340B program helps hospitals, community health centers and other covered entities to purchase outpatient drugs at discounted prices. Well, these are not for personal profits, and the aim of the program is to get the savings to support expanded patient care and community services.
Now, owning a healthcare center that comes under 340B program is already a huge responsibility. And for multi-clinic entities, the program brings a different set of challenges. 340B reporting becomes more complex for multi-clinic entities when managing multiple child sites, extensive 340B contract pharmacy networks, and compliance rules tied to 340B ESP submissions and 340B medicaid billing. It is important to be extremely careful and compliant as errors in reporting may lead to audit findings, financial penalties, or loss of eligibility for the program.
Did you know that in 2023, discounted purchases under the 340B program reached a record $66.3 billion, representing a 24% year-over-year increase? Also, covered entities have also expanded significantly, with more than 60,000 participants enrolled as of early 2025, a 600% increase since 2000. This scale shows both the importance of the program and the need for accurate, transparent reporting to maintain compliance.
This blog outlines how multi-clinic organizations can streamline 340B reporting by focusing on:
- Centralized governance
- Technology adoption
- Workflow optimization
- Advanced compliance strategies
It also explains how GATP Solutions provides accounting expertise and smart 340B reporting solutions to make this process more efficient for your organization.
Establish Centralized Program Governance
An effective governance is the base of a strong and reliable 340B reporting. If you are a multi-clinic entity, you need to create an oversight structure for consistency, accountability and reduce mismanagement across different locations. Here is what you can do to ensure proper governance:
Build a Multi-Disciplinary Oversight Committee
A dedicated oversight committee ensures alignment across all child sites and 340B program pharmacy partners. It should include leaders from finance, compliance, IT, and pharmacy. By meeting regularly, this team reviews reporting performance, ensures data integrity, and keeps all departments prepared for HRSA audits.
Standardize Policies and Procedures
Multi-clinic systems often struggle with fragmented processes across different sites. If you standardize the policies and procedures, it will eliminate any possible inconsistencies by making it mandatory for all the clinics to follow the same rules and regulations. This not only simplifies reporting but also ensures that audits can be managed with a single framework.

Implement Technology Solutions
Manual management is long gone and is now a thing of the past. Technology provides the tools to manage the complexity of multi-site programs. For large organizations, manual reporting is no longer sustainable, making automation and system integration essential. Here are some of the options you can explore to implement technology solutions to your procedure:
Enterprise-Wide 340B Management Software
- Provides administrators with visibility into all sites from one dashboard
- Consolidates claims across multiple entities
- Automates mapping of charge description masters to drug codes
- Offers an enterprise-wide view of compliance
- Builds confidence in reporting accuracy and efficiency
Automate Data Integration and Workflow Management
- Reduces manual workload and improves accuracy
- Reconciles claims across TPAs, billing systems, and EHRs automatically
- Uses AI tools to monitor diversion risks and duplicate discounts
- Ensures cleaner and more consistent 340B ESP submissions
- Validates data automatically to minimize rejections and protect program savings
Optimize Reporting Workflows
Once governance and technology are sorted, organizations must fine-tune their workflows to ensure reporting consistency across all locations. This step is especially important for entities with large networks of contract pharmacies. Following are the steps to optimize your reporting workflows:
Consolidate Data Collection and Validation
To strengthen reporting, multi-clinic entities should consolidate the collected data into a single system. This means requiring clinics and 340B contract pharmacy partners to use standardized formats for patient eligibility, encounter coding, and claims documentation. Also, if you have monthly reconciliations of purchases against the dispenses, it can help you identify any discrepancies before they impact compliance.
Centralized Audit Preparation
Audits are one of the major tasks while dealing with the 340B compliance. If there are any issues found during audits, it may create troubles for your organization. Hence, audit preparation should be built into everyday workflows. By having a centralized repository for 340B documentation, it makes it easier to showcase compliance during the HRSA audits.
Use Advanced Compliance Strategies
Compliance strategies must go beyond periodic audits as they ensure consistent checks and adherence to the rules. To stay compliant, multi-clinic entities need ongoing monitoring and proactive risk management to strengthen integrity across the enterprise. Here are some of the compliance strategies you can use:
Continuous Monitoring Systems
By using continuous monitoring systems, you get access to real time KPIs that allow the administrators to track compliance across every site. Check metrics like the audit readiness, claims qualification rates, and issue resolution times to get proper visibility and take quick actions over any anomalies.
Optimize Contract Pharmacy Networks
For organizations managing a wide 340B contract pharmacy network, performance must be closely monitored. By using data-driven analysis, you can evaluate pharmacy partners better and understand any underperforming relationships. Also, centralizing oversight of TPAs ensures reporting practices are consistent across all contract pharmacies. And by ensuring automated referral capture, you can receive prescriptions that are properly recorded, protect savings and strengthen compliance.
Address State-Specific Reporting Requirements
State reporting adds another layer of complexity for multi-clinic entities while taking care of 340B reporting and compliance. These requirements often vary by jurisdiction and need special attention before diving into details.
State Reporting Challenges
The following are the most common state reporting challenges faced by multi-clinic entities while reporting for the 340B program:
- Multi-clinic entities face added responsibilities when states require 340B Medicaid reporting beyond federal rules.
- Organizations must adapt reporting systems to meet different standards across jurisdictions.
Automated State-Level Reporting Tools
By using automated state-level reporting tools, you can:
- Allow quick preparation of state-required reports with fewer errors.
- Provide a single source of truth for both state and federal reporting.
- Reduce redundancy while ensuring full compliance across multiple jurisdictions.
Establish Performance Metrics and KPIs
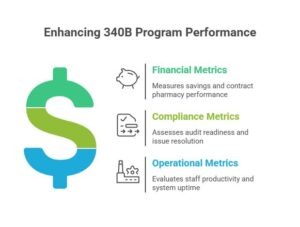
For every organization, it is important to monitor the KPIs to ensure compliance across finances, operations, and the workflow. These measurements help ensure accountability and gives you the chance to take care of any possible issues and make improvements in your 340B reporting. The following are the metrics multi-clinic entities should track:
- Financial Metrics: Track savings by site, evaluate contract pharmacy performance, and monitor WAC spend reduction.
- Compliance Metrics: Measure audit readiness, claims qualification rates, and issue resolution times.
- Operational Metrics: Assess staff productivity, data feed accuracy, and system uptime.
By monitoring these areas, multi-clinic entities can strengthen program performance while maintaining compliance.
GATP Solutions: Partner for Streamlined 340B Reporting
GATP Solutions provides healthcare organizations with the accounting expertise and technology needed to simplify 340B reporting. For multi-clinic entities, GATP’s software-driven approach integrates compliance, finance, and operations into one seamless process.
Our solutions help providers:
- Automate reconciliations across 340B program pharmacy networks.
- Build audit-ready workflows for HRSA and manufacturer reviews.
- Manage compliance for 340B ESP submissions and 340B medicaid billing.
- Deliver dashboards that offer enterprise-wide visibility into financial and compliance outcomes.
As Rick Pollack, President and CEO of the American Hospital Association, has emphasized, “The 340B Program was created to help hospitals reach more eligible patients and provide more comprehensive services. 340B is vital in advancing health in communities across the country.” At GATP Solutions, we share this vision by helping providers use strong reporting systems to ensure that savings directly support better patient care.
By combining technology with accounting expertise, GATP Solutions helps multi-clinic entities reduce administrative burden, safeguard compliance, and maximize 340B savings.
Conclusion
For multi-clinic entities, 340B reporting is one of the most complex aspects of the program. Multiple sites, extensive contract pharmacy networks, and state-level compliance add significant challenges.
By building strong governance structures, implementing enterprise-wide technology, standardizing workflows, and embracing continuous compliance monitoring, healthcare providers can simplify reporting and protect program integrity.
With expertise in both accounting and technology, GATP Solutions partners with healthcare organizations to transform 340B reporting from a manual, error-prone process into a streamlined system that maximizes savings and supports patient care.
Frequently Asked Questions
- What is 340B reporting and why is it important for multi-clinic entities?
340B reporting refers to the process of documenting and reconciling all drug purchases, dispenses, and claims made under the 340B Drug Pricing Program. For multi-clinic entities, accurate reporting is critical to maintain compliance with HRSA rules, avoid duplicate discounts in 340B medicaid billing, and ensure that savings are reinvested in patient care.
- How does a 340B contract pharmacy impact reporting requirements?
A 340B contract pharmacy adds complexity to reporting because covered entities must track claims from external pharmacy partners alongside their own clinics. Multi-clinic entities often manage dozens of contract pharmacies, making centralized data collection and reconciliation essential.
- What role does 340B ESP play in reporting?
340B ESP is a data submission platform where covered entities report contract pharmacy claims to manufacturers. Clean, automated submissions reduce errors and ensure access to discounted pricing. Multi-clinic organizations should integrate ESP reporting into their broader compliance strategy.
- Why is Medicaid billing compliance critical in 340B reporting?
340B medicaid billing requires strict controls to prevent duplicate discounts. Entities must coordinate with state Medicaid agencies, use exclusion files correctly, and maintain audit-ready documentation. Non-compliance can result in penalties or removal from the program.
- What are the main challenges in 340B reporting for multi-clinic entities?
- Consolidating data from multiple clinics and pharmacies
- Managing complex 340B contract pharmacy networks
- Preventing duplicate discounts in 340B medicaid billing
- Submitting clean claims to 340B ESP
- Preparing for HRSA and manufacturer audits
- How can technology streamline 340B reporting?
Enterprise 340B software automates claim reconciliation, provides dashboards for multi-site oversight, and standardizes data feeds. Automation also supports real-time monitoring and simplifies submissions to platforms like 340B ESP.
- What are best practices for audit preparation in 340B reporting?
- Maintain a centralized repository for all 340B records
- Standardize data across child sites and 340B program pharmacy partners
- Reconcile purchases and dispenses monthly
- Prepare crosswalks that connect cost reports, trial balances, and EHR data
- How often should multi-clinic entities reconcile 340B data?
Monthly reconciliation is considered best practice. This ensures that discrepancies are identified early, prevents compliance issues, and supports smooth 340B reporting during HRSA or manufacturer audits.
- What KPIs should healthcare systems track for 340B reporting?
- Financial metrics: savings per site, WAC spend reduction, contract pharmacy performance
- Compliance metrics: claims qualification rates, audit readiness scores
- Operational metrics: data feed accuracy, staff productivity, system uptime
- How does GATP Solutions help with 340B reporting?
GATP Solutions provides software-driven accounting tools that automate reconciliations, ensure accurate 340B ESP and 340B medicaid reporting, and build audit-ready workflows. With dashboards for enterprise-wide visibility, GATP enables multi-clinic entities to simplify reporting while protecting compliance and maximizing savings.